- Non Conformance Procedure Pdf
- Control Of Non Conformance Procedure
- Non Conformance Procedure Food Industry
- Non Conformance Investigation Procedure
A non-conformance (or 'nonconformity') means that something went wrong.
Handling of Non-Conformance Procedure Non-conformance (NC) is a deviation, variation, or disparity from a set of agreed standards, specifications, procedures, or instruction mutually agreed upon by Contractor and Employer for the benefit of the implementation of a Quality Management System. Nonconforming Product. Consumer Safety Officer. Postmarket and Consumer Branch. Division of Industry and Consumer Education. Office of Communication and Education.
The non-conformance could be in a service, a product, a process, goods from a supplier, or in the management system itself. It occurs when something does not meet the specifications or requirements in some way. These requirements might be defined by the customer, a regulatory body, or in the internal procedures of the company.
Here are some examples:
- You manufacture steel plates with a hole of size 10mm, but on inspection the hole measures 9mm.
- You supplied product to a customer that was not the colour they ordered.
- As a labour hire company you sent a worker to a client without the correct induction.
- Your consulting office sent a report to a client that is missing a section of work
- Your testing company misplaced some client samples
- A supplier has sent you the wrong product
- Your batch records are missing temperature information and the supervisor's signature
- Sales staff are sending out an old version of a product specification sheet
- You shipped product to a customer but sent it to the wrong address
- Your help desk did not respond to a customer within the 24 hour response time target
These issues could be identified through customer complaints, internal audits, external audits, incoming material inspection, or simply during normal testing and inspection activities.
Nonconformity is addressed with corrective actions and they are both in the same clause in ISO 9001:2015 (10.2).
ISO 9001:2015 no longer requires a documented procedure, but you must still keep records ('retain documented information') of the nonconformity and what was done to correct it.
You must establish a process (documented or not) for how your organisation will deal with non-conformance and how you will keep records of what happened.
If you make and/or sell goods, then you also need to work out how to deal with nonconforming product. The highest priority is to stop nonconforming goods reaching the customer, but the sooner you detect a problem, the less it will cost you.
In establishing a process for dealing with nonconformity you'll need to decide:
- who will determine what immediate actions will be taken to correct the problem, and what kinds of actions should be taken. These immediate corrective actions can be seen as 'damage control' and need to:
- stop further non-conformance
- contain the effects and stop any further processing of defective items- e.g. quarantine
- assess the effects of the problem - how much, how bad, what to do (e.g. scrap / rework),
- notify affected customers, if necessary
- how reworked items should be checked (if it is different from normal inspection)
- how and where a non-conformance should be recorded
- what steps should be taken to identify any defective product released to a customer
- what, if any, concessions/discounts will be given to the customer
- how a decision will be made on whether further corrective action is necessary
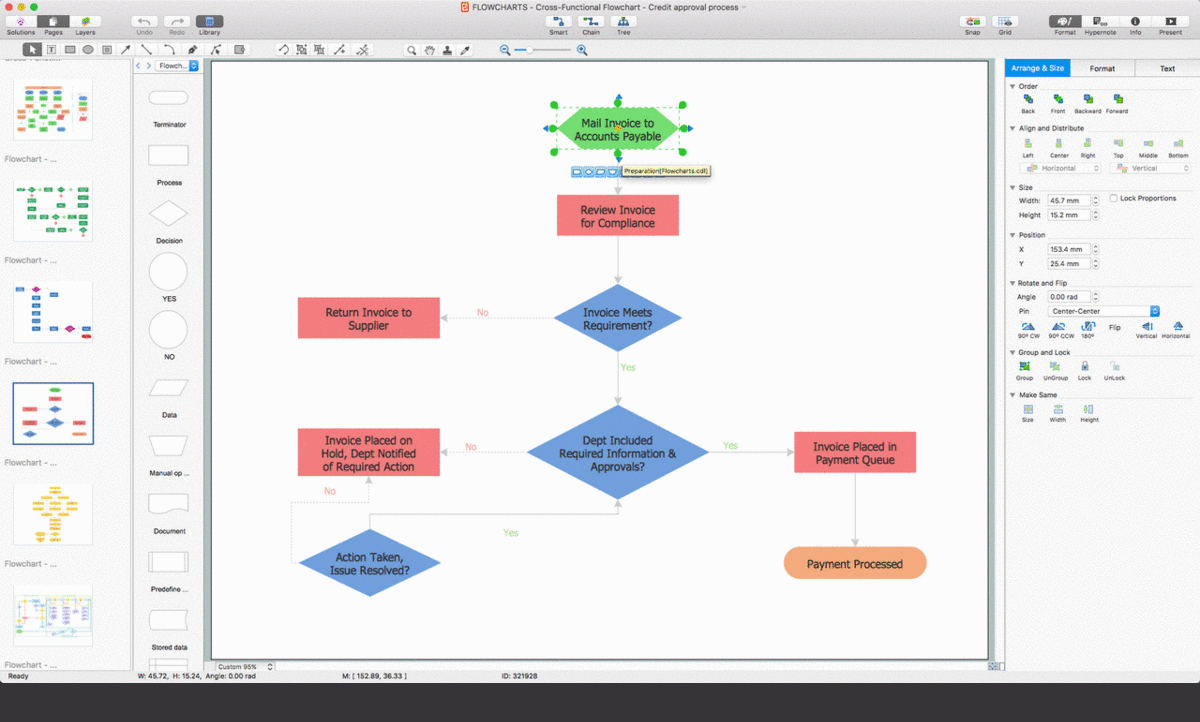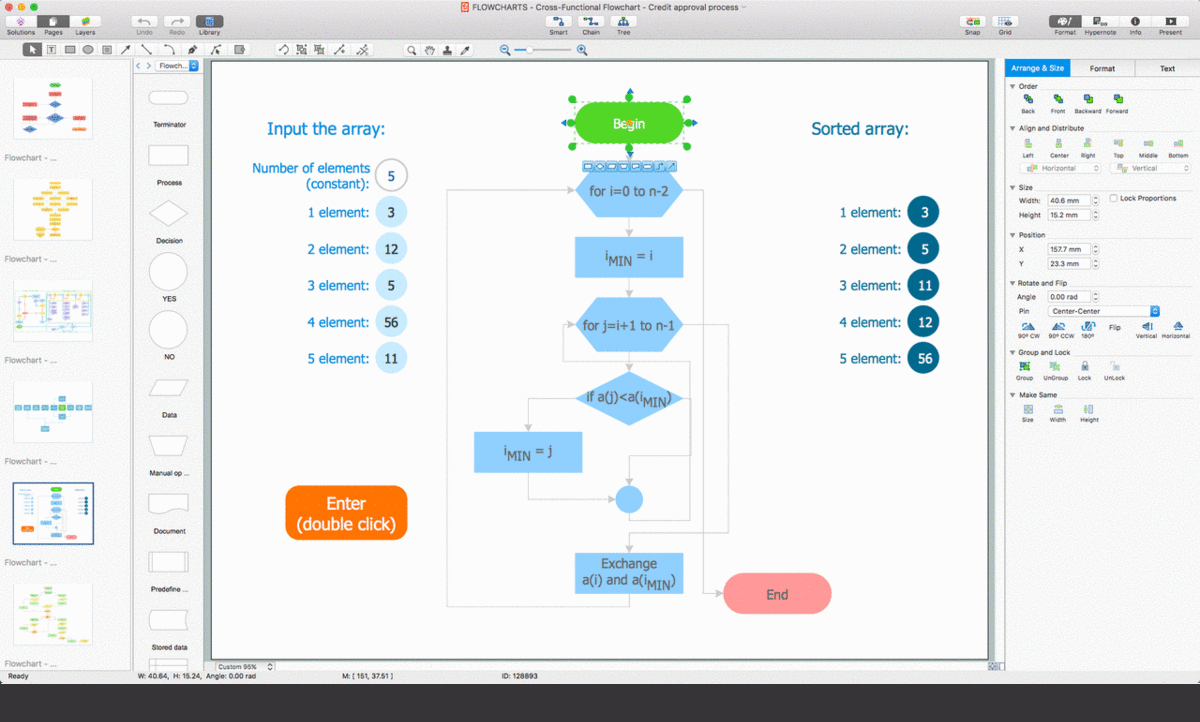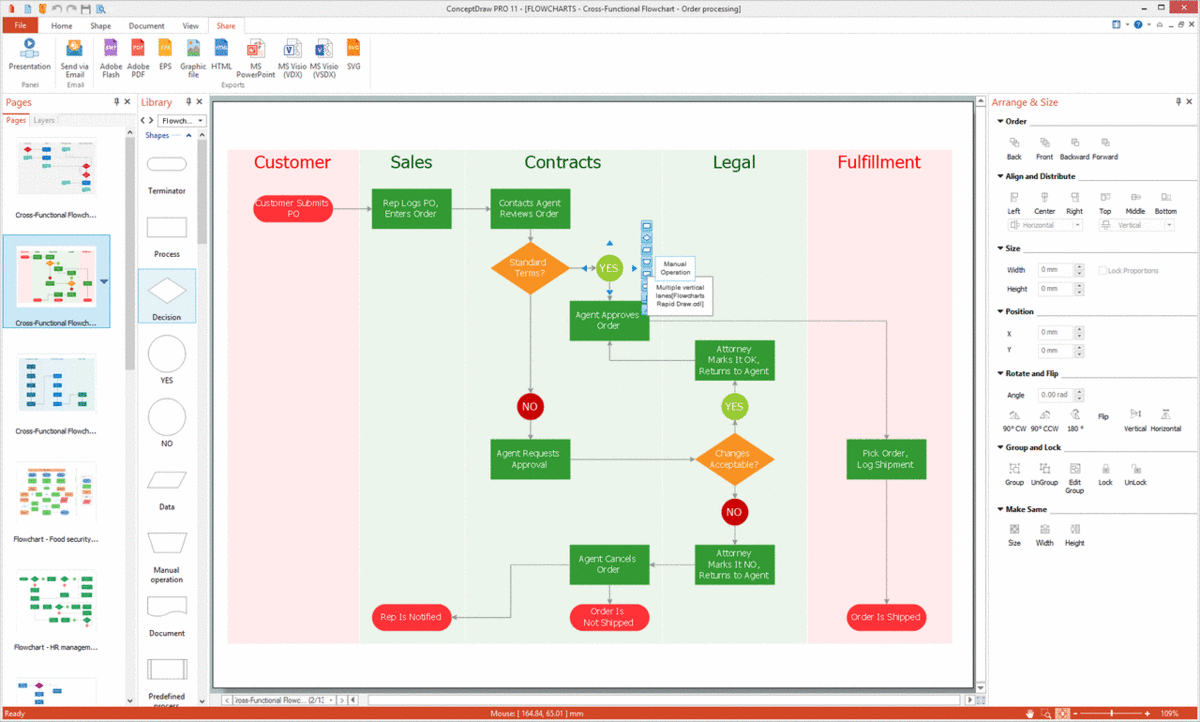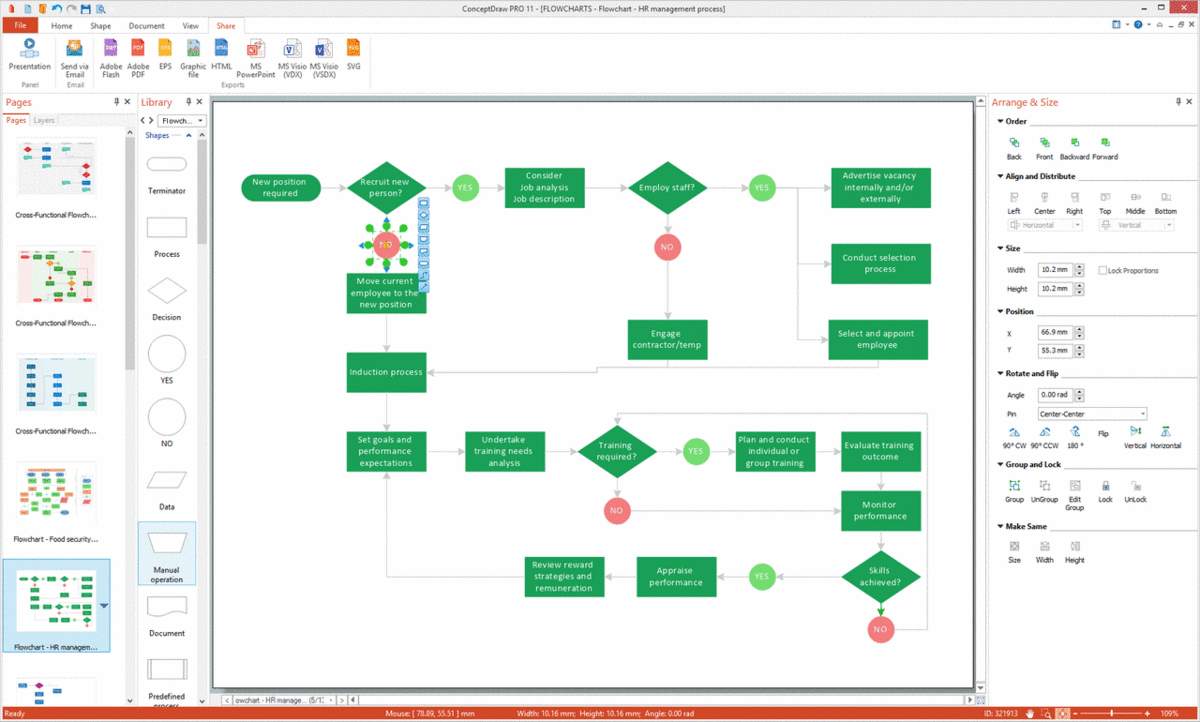
A basic process for manufacturing might go something like this:1. All staff are responsible for reporting a nonconformity to their supervisor.1. Staff or supervisor must fill out Form-99. *1. Also add details to the NCR register and record the assigned NCR number on Form-99. *1. Check any previous production to ensure conformity. Follow recall process if any defective product was released to customers1. Move all affected goods to the designated quarantine area and attach a 'non-conforming goods' label1. Investigate the source of the problem and correct it before resuming production.1. Supervisor to determine what action should be taken with nonconforming goods - rework, scrap, etc,1. Supervisor to record decision on Form-99. *1. If the scheduled delivery date will be affected, inform the customer1. Reworked items will be checked as per normal inspection with doubled sampling rates1. See corrective action for more details on what else needs to happen to address a nonconformity, i.e. actions to investigate and eliminate the root cause(s).1. All corrective actions to be recorded on Form-99. *1. When root cause has been addressed and verification of effectiveness has been completed, manager to review and sign-off NCR as closed on NCR register. *1. Manager and supervisor to review NCR register on a monthly basis and followup outstanding tasks.
* Toolbox software eliminates the need for either form or register, since both are taken care of within the software. Toolbox gives you a process to follow AND a place to keep the records for both non-conformance and corrective action.
In Toolbox software, nonconformities are recorded as 'Issues'. The same process is used to manage customer complaints, supplier problems, audit findings, improvement requests, maintenance issues, document change requests, and any number of other issue types you'd like to configure. Have a look in our user guide to see how you would report a nonconformance, and what the corrective action process looks like in Toolbox.
Non Conformance Procedure Pdf
Control of Nonconforming Output Procedure
GENERAL REQUIREMENT
This procedure provides guideline to extent information explained in clause 8.7 Control of Nonconforming output of the Quality Manual.The requirement is also enable to provide conformity to clause 8.7.1 and 8.7.2 of Control of nonconforming outputs of ISO 9001:2015 Standard Requirement.
COMMON DEFINITION USED
The definitions addressed are mainly refer to IS0 9000:2015Control Of Non Conformance Procedure
RESPONSIBILITY AND AUTHORITY
IDENTIFICATION OF NON-CONFORMITY
Nonconformity of output in the [scope of activity] activity can be identified throughout one or combination of following occurrence;
Non Conformance Procedure Food Industry
- Inappropriate planning as described in clause 8.1 Project Planning of this Quality Manual
- Defect detected by the personnel during construction where it does not meet with specification as defined in clause 8.5.1 Project management control of this Quality Manual
- Absence of competent person if it is required by the clause 7.2 Competence of this Quality Manual
- Defective purchased material or out of specification material as defined in clause 8.4 Control of externally provided processes, products and services of this Quality Manual caused by supplier.
- Other potential undesired consequences associated with services (example; measurement faulty caused by unfit measurement instrument as per clause 7.1.5 Monitoring and measuring resources of Quality Manual)
- Customer complaint
- Complaint received during warranty period
CONTROL OF NONCONFORMITY
Non Conformance Investigation Procedure
- Whenever nonconformity is detected;
- Nonconformity with regard to the facility or material or physical property, the On-Hold tag must be placed to the defect unit accordingly.
- If related to the human resources, proceed to the clause 7.2 Competence of Quality Manual.
- Review possibility of occurrence due to weaknesses of communication factor as described in clause 7.4 Communication of Quality Manual
- The nonconformity shall be reviewed by
for disposition, which may be any of the following; Hold or must not to be used, or Concession by authorized person The concession of nonconformity is not accepted for concession if high impact to the quality issues or jeopardize the company reputation as defined in Risk Analysis. Results of the review shall be recorded by on the CPAR form The shall be responsible to review the nature and seriousness of nonconformity. For nonconforming condition that have been accepted by concession when regulatory requirements are met. The record of the identity of the person(s) authorizing the concession shall be maintained. If action will be taken other than decision stated in #2, the non-conformity shall be corrected. will verify the measures taken before release non-conformance status. Designated person will decide whether the issuance of CAR form, the nonconformity need further investigation throughout the Corrective Action Procedure or used as record purpose only. For purchased material/part which are found defective or out of specification, [designated person] shall arrange to return the item to the supplier for replacement or further action. CPAR form should be issued. Any defective purchased material requested for concession, the process should follow step as described in the #9. Top management will decide for necessity to inform the nonconformity depends on the severity of the issues. QMR shall maintain CPAR status log. Status of CAR shall be highlighted for management review meeting.